Instrument Bells Bleck

| Price: | 295 UAH |
| Company: | ChP Ladyika |
| Seller: |  Oleg Zulyak Nikolaevich Oleg Zulyak Nikolaevich |
| Phones: |  380684413077 Show phone |
| Address: | Ukraine, Ternopil's'ka Oblast', Ternopil |
It is based on the recovery of arsenic compounds to the arsenious hydrogen. Last depending on the amount of arsenic stains the paper treated with the bromide of mercury or mercury dichloride (corrosive sublimate), from yellow to dark brown.
As a result of the arsenic reaction of hydrogen with dichlo-reed mercury forms a complex compound Ag(HgC1)3, Hg2Cl2, giving staining.
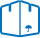
The course of the analysis. For the detection of arsenic using device, bells bleck, which consists of reducing cones with a capacity of 100 ml, tube with cone and ball-shaped extension at the bottom and the vapor tube.
In a spherical expansion lay the wool, previously soaked in 5% solutions of lead acetate and dried. In the upper part of the tube is placed a piece of filter paper impregnated with 5% solution of bromide of mercury or of mercuric chloride and dried.
In the reducing flask introduce 20 grams of powdered test material, 5-6 granules of zinc metal and 5-10 mg (the tip of a knife) tin chloride. Then add 40 ml of sulfuric acid diluted in the ratio 1:8, quickly cover the head and put in a fume cupboard for 1 h.
In the presence of hydrogen ARSENICAL sublimate a piece of paper changes color from light brown to black.
After the reaction, the reactive circle of paper is dipped first in a saturated solution of iodide of cadmium, and then in a saturated solution of potassium iodide, then washed with water. Clear, brown or black coloration confirms the presence of arsenic.
When using reactive paper impregnated with bromide of mercury, they can not exhibit the iodide of potassium.